With thanks to the Health & Safety Executive (HSE) for their patience in explaining all the rules to us, Pest has managed to unravel the actual use-by dates for products affected by the EU Article 95. And, the good news is that you generally have longer than the manufacturers or distributors thought.
With just four working days before all the products affected by this EU regulation can no longer be sold, it might pay you to ring round and buy in some extra stock of any you use regularly. There are some good deals to be had and our research has revealed that there’s even longer to use up these stocks than distributors and manufacturers thought.
| To help our readers, HSE has made it easy by providing us with use-by dates for all the key products affected by Article 95. Manufacturers have also contributed with Hockley pointing out that the manufacturing expiry date on all their products is 31 August 2016, so several months earlier than the regulatory use-by dates. Download and keep a copy of our PDF table on Article 95 withdrawn insecticides use-by dates.
Variety of use-by dates published It’s not that the companies have been trying to be difficult, it’s just that interpreting the rules that have come out of Brussels is not easy – read our article Product withdrawals: What a muddle! in Pest Issue 40: August & September 2015 Don’t forget though that if you do stock up you must comply with all the regulations on storage. So why the confusion and what’s the HSE’s interpretation of the rules? Well, it’s a bit complicated so take this slowly. There are two bits of regulation involved, Article 95 and Article 89 and this is where the confusion has come in. Article 89 is designed to allow for the transition from the old approval system – the UK Control of Pesticides Regulations (COPR) – to the new EU approval system under the Biocides Regulation (known as BPR). The relevant sub clauses being (2), (3) and (4) where it sets ‘sell-by’ and ‘use-by’ deadlines for products containing active substances that have gone through the EU BPR review. |
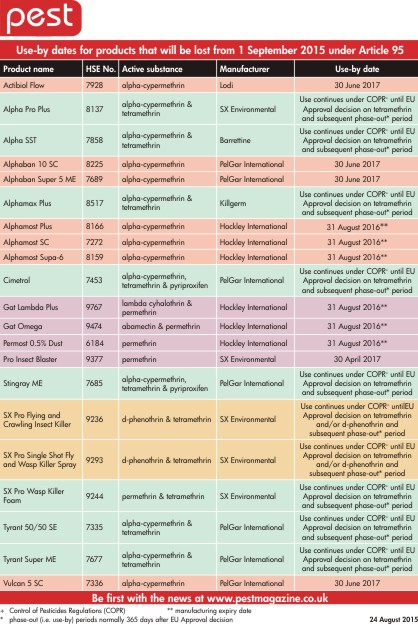
Download the Article 95 withdrawn insecticides use-by dates table
Some of the insecticide products affected by Article 95 |
|
| If an active substance receives a non-approval decision, the sale of affected products must cease within 12 months of that decision and use within 18 months.
However, if an active substance receives a positive approval decision, an official Approval date will be set at EU level. This will be at a time in the future to give companies time to put together applications for country-specific product authorisations. These must be submitted by the official Approval date. In the UK product authorisation applications go to the HSE. Without going into all the detail, it is extremely unlikely that the companies making the products now affected by Article 95 will decide to apply for product authorisation. Where no product authorisation is applied for then Article 89 gives sellers 180 days to continue to sell the products and users have 365 days to use them up. These periods run concurrently and start from that official EU active substance Approval date. Article 95 is purely concerned with ‘the making available on the market’ i.e. selling of certain products. It overrules the normal ‘sell-by’ dates that would apply under the Article 89 transition arrangements, effectively bringing the date forward so that no Article 95 affected products can be sold after midnight on 31 August 2015. But as HSE has explained to Pest: “Article 95 does not have any effect on use. Use continues as it would always have done under Article 89.” In practice this means there is not one date when all the affected products must be used by. Their use-by dates depend on the BPR status of the active substances they contain and the transitional arrangements for those active substances under Article 89. All this is much easier to follow in some examples
Still confused? |
||




